Selectivity In Catalysis
- Introduction
- Regioselectivity in the Dehydrogenation of Alkyl Surface Species
- C=C Double Bond Migration
- Cis-Trans Isomerization in Olefins
Introduction
Building on our extensive past work on the mechanism of hydrocarbon conversions on transition metal surfaces, we continue to address issues of selectivity for those reactions [F. Zaera, J. Phys. Chem. B 106 (2002) 4043; F. Zaera, Acc. Chem. Res. 42 (2009) 1152]. Selectivity is one of the most important criteria for the design of new catalytic processes. More selective catalysis could be both cheaper and greener because it does not waste reactants, does not require expensive separation procedures, and generates fewer toxic byproducts. Traditionally, control of selectivity in heterogeneous catalysis has been hampered by both a lack of understanding of the molecular details that define such selectivity and the limited range of synthetic tools available to make catalysts with the specific properties required. However, progress in surface science as well as in nanotechnology and self-assembly are providing greater molecular understanding and a wider synthetic range to address these limitations.
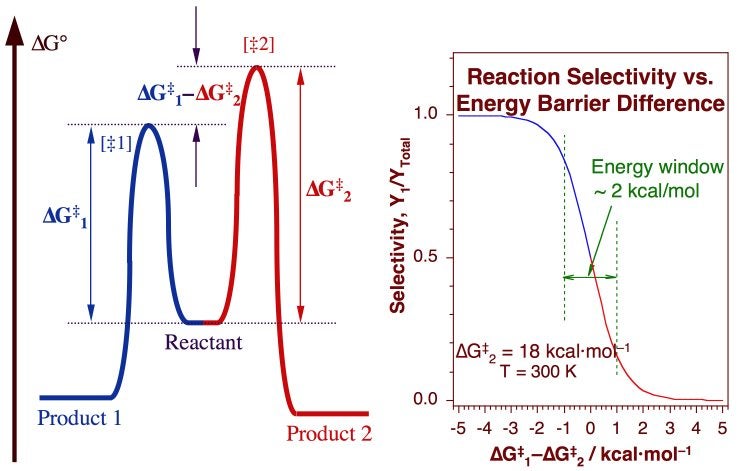
The success of catalysis relies on the ability of the catalyst to modify the kinetics of the chemical system being catalyzed. Specifically, a catalyst accelerates chemical reactions by opening alternative mechanisms with lower activation barriers. When considering selectivity, however, what matters is not so much the absolute height of the barrier towards the desired pathway, but its relative height compared to those of other undesirable side steps (Figure 1). Controlling the relative heights of activation energy barriers is a subtle and difficult task that requires a better understanding of the underlying chemical principles behind catalytic reactions.
Illustration of the key role that relative heights among activation barriers for different reactions play in determining the selectivity of catalytic processes. The diagram on the right depicts a one-dimensional potential energy surface for the conversion of a reactant to two possible products. The rates of those reactions are determined by the absolute activation barriers, indicated here as deltaG1 and deltaG2, but the selectivity between the two is controlled by the difference between those two values. The calculated selectivity of this two-reaction system, plotted as a function of deltaG1 - deltaG2 in the left side of the figure, shows how a relative variation of about 10% in absolute barrier heights can lead to the switch in selectivity from the exclusive formation of one product to the other. We believe that being able to control selectivity is the main issue facing catalysis in the future.Regioselectivity in the Dehydrogenation of Alkyl Surface Species
Much of our work in this area has focused on dehydrogenation steps within hydrocarbon surface species. In our initial work, described in the Section entitled "Hydrocarbon Reforming", we have looked into the regioselectivity of hydrogen removal from adsorbed alkyl species. The main lesson that derived from those studies is that the dehydrogenation of adsorbed alkyl moieties can take place at different positions within their molecular structure, at the alpha, beta, or gamma carbons of the hydrocarbon chain (as they are labelled in order of distance away from the surface), and that removal of a hydrogen atom from each of those positions leads to different products [F. Zaera, Catal. Lett. 91 (2003) 1; F. Zaera, Chem. Rev. 95 (1995) 2651; Z. Ma, F. Zaera, Surf. Sci. Rep. 61 (2006) 229]. Our early work indicated that, by far, the faster dehydrogenation step involving alkyl adsorbates is from the beta position, the same as in organometallic chemistry [F. Zaera, J. Am. Chem. Soc. 111 (1989) 8744]. This step leads to the formation of alkenes, and to fast alkane-alkene equilibria in catalysis. However, dehydrogenation steps at other positions, albeit slower, are required to promote most other catalytic reactions.
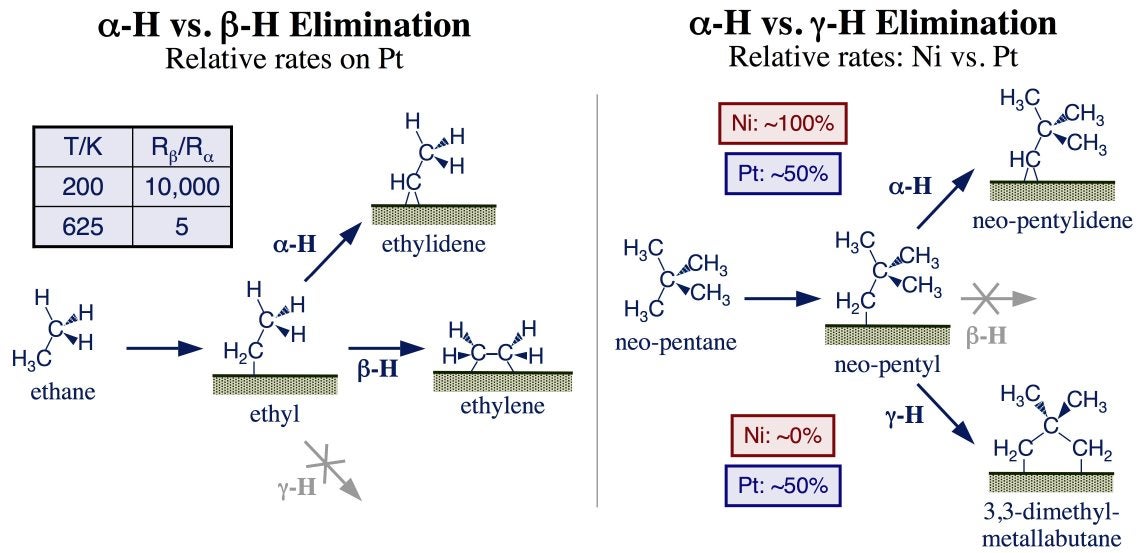
Rates for alpha-H elimination have been measured directly from methyl moieties [F. Zaera, Langmuir 7 (1991) 1998; S. Tjandra, F. Zaera, Langmuir 8 (1992) 2090; C. J. Jenks, B. E. Bent, F. Zaera, J. Phys. Chem. B 104 (2000) 3017], but have also been estimated relative to beta-H elimination from ethyl species [A. Loaiza, M. Xu, F. Zaera, J. Catal. 159 (1996) 127; F. Zaera, S. Tjandra, T. V. W. Janssens, Langmuir 14 (1998) 1320]. On platinum, as on most metals, elimination from the beta position is several orders of magnitude faster, but less so at higher temperatures: at 625 K, a temperature within the range of those used in many catalytic processes, the rate for alpha-H and beta-H eliminations differ by only a factor of five (see Figure below, left). Additional comparisons between alpha-H and gamma-H eliminations using neopentyl intermediates indicated that on nickel alpha-H elimination, a step believed to lead to undesirable C–C bond-breaking and hydrogenolysis reactions, dominates [F. Zaera, S. Tjandra, J. Am. Chem. Soc. 118 (1996) 12738], whereas on platinum the rates of both steps are comparable (Figure below, right) [T. V. W. Janssens, G. Jin, F. Zaera, J. Am. Chem. Soc. 119 (1997) 1169; T. V. W. Janssens, F. Zaera, Surf. Sci. 501 (2002) 16]. This is likely to be the reason why platinum is a particularly good catalyst for reforming catalysis.
Two mechanistic examples of regiospecificity in hydrocarbon dehydrogenation reactions on metal surfaces. Left: Comparison between alpha-H and beta-H elimination from ethyl groups bonded to Pt(111) surfaces. In general, the latter is faster than the former, but, because elimination from the alpha position displays a higher activation energy, the difference becomes smaller at the temperatures typically used in hydrocarbon reforming. Right: Contrast between alpha-H and gamma-H elimination steps. The former clearly dominates on nickel substrates, whereas the rates of both are comparable on platinum surfaces.The general picture that emerges is one where the nature of the catalyst plays a central role in determining selectivity. Most transition metals are good dehydrogenation catalysts, but promote alpha-, beta-, and gamma-H eliminations with different relative efficiencies; that is what really matters in terms of selectivity. For instance, nickel is a particularly good promoter of undesirable alpha-H elimination steps, whereas platinum is comparatively efficient promoting the gamma-H elimination steps that lead to desirable alkane isomerizations. It may in fact be possible to use the correlation identified between this selectivity and the %d character of the metal [Zaera, Chem. Rec. 5 (2005) 133] to fine-tune dehydrogenation catalysis via alloying. The structure of the surface may also contribute to define the relative rates of these dehydrogenation steps, but if so, that is a less marked effect [F. Zaera, Appl. Catal. A 229 (2002) 75].
C=C Double Bond Migration
A subtler example of regioselectivity is seen in the case of the migration of carbon-carbon double bonds in alkenes. The first step in that reaction is the incorporation of a hydrogen atom into one of the two carbons of the double bond in the alkene and the formation of an alkyl surface intermediate; the alkyl is then converted back to an alkene via a beta-H elimination step. The thing to notice here is that since the alkyl intermediate may contain different types of beta hydrogens, that is, since it may have hydrogens bonded to beta-carbon atoms in different chemical environments, the beta-H elimination may not necessarily involve the same hydrogen incorporated in the preceding half-hydrogenation step. If that is the case, a new molecule may be made.
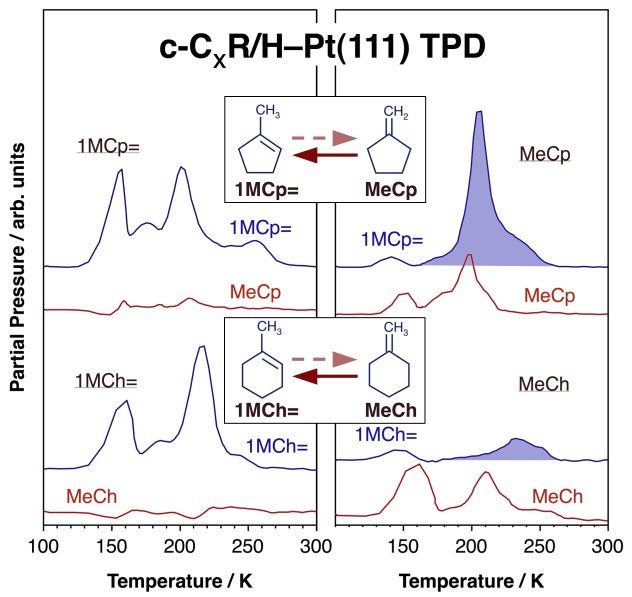
One example of this type of isomerization is the migration of C=C bonds in cyclic compounds. The Figure below summarizes the temperature-programmed desorption (TPD) data obtained for two systems involving C5 [R. Morales, F. Zaera, J. Phys. Chem. B 110 (2006) 9650] and C6 [I. Lee, F. Zaera, J. Am. Chem. Soc. 127 (2005) 12174] cyclic moieties, respectively. Both reactions show preferential migration of the double bond from the exo position into a carbon-carbon bond inside the cycle. This is because the outside carbon in the methylene group of both methylenecyclopentane and methylenecyclohexane is the easiest to hydrogenate, and because beta-H elimination from an inner carbon in the ring of the resulting methylcyclopentyl and methylcyclohexyl surface intermediates, respectively, is favored over the reversal of the original hydrogenation step. The difference in the activation barriers of the two dehydrogenation pathways available to the methylcycloalkyl intermediate is only about 3 kcal/mol, but that is sufficient to obtain high selectivities towards the production of the methylenecycloalkane.
Temperature programmed desorption (TPD) data from studies on the regiospecificity of carbon-carbon double bond migration reactions on Pt(111) surfaces. Two examples, both involving cyclic compounds, are provided, namely, the interconversions between 1-methylcyclopentene (1MCp=) and methylenecyclopentane (MeCp; top traces), and between 1-methylcyclohexene (1MCh=) and methylenecyclohexane (MeCh; bottom traces). Traces are shown for the desorption of both compounds when starting with surfaces covered with either the methylcycloalkenes (left panel) or the methylenecycloalkanes (right panel). In both cases, preferential migration is seen toward the former compounds, that is, toward migration of the double bond to a position within the cyclic moiety (shaded peaks).Cis-Trans Isomerization in Olefins
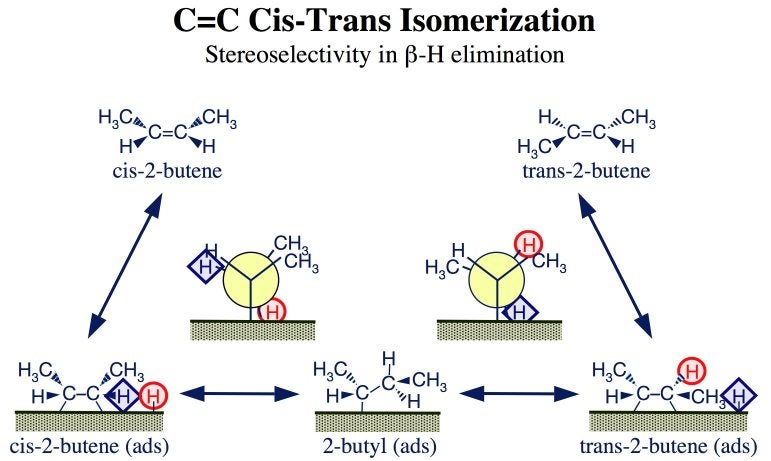
An even subtler example of selectivity involving beta-H elimination from alkyl intermediates was identified in the cis-trans isomerization of C=C bonds in alkenes. This is a problem we have been investigating in great detail recently. The mechanism involved is illustrated in the Figure below for the case of 2-butenes. As in double-bond migrations, C=C cis-trans isomerizations require an alkene hydrogenation step and a subsequent beta-H elimination from the resulting alkyl surface intermediate. However, here, the latter reaction always occurs at the same beta carbon atom, also the same involved in the initial hydrogenation (the inner carbon not bonded to the surface). Selectivity, therefore, does not come from the regiospecificity of the dehydrogenation step, but rather from the steric differences in the configurations required to abstract the different hydrogens within the same beta carbon atom. This is better illustrated by the Newman projections in the Figure below, where the two hydrogens bonded to the inner beta carbon have been labeled with a red circle and a blue diamond, respectively: elimination of the red-circle hydrogen leads to the production of cis-2-butene, whereas elimination of the blue-diamond hydrogen results in the formation of trans-2-butene.
Mechanism of the interconversion between the cis and trans isomers of 2-butene on metal surfaces. This reaction goes through a common 2-butyl intermediate, and proceeds in a direction defined by the relative stereo restrictions imposed on the transition state of the two possible beta-H elimination steps from that moiety (as indicated by the Newman projections in this figure).In our initial surface-science work on these systems we found that, on Pt(111) surfaces, trans-2-butene isomerizes preferentially to its cis counterpart [I. Lee, F. Zaera, J. Am. Chem. Soc. 127 (2005) 12174; I. Lee, F. Zaera, J. Phys. Chem. B 109 (2005) 2745: I. Lee, F. Zaera, J. Phys. Chem. C 111 (2007) 10062]. That is a surprising result, not what is expected thermodynamically, and also not what is commonly observed in catalytic processes. Certainly, significant amounts of trans fats are produced during the partial hydrogenation of edible oils, a fact that has gained national attention because of the associated adverse health effects. This is because the catalytic hydrogenation of olefins is often accompanied by cis-trans isomerization reactions, since they both share the same surface alkyl intermediates. Natural oils are almost exclusively comprised of cis olefins, but the fat obtained after their partial hydrogenation almost invariable contains a large fraction of (undesirable) trans C=C double bonds.
A more detailed study of this system revealed a couple of key factors that affect selectivity in cis-trans conversions of alkenes. Perhaps first and foremost is the effect exerted by coadsorbed hydrogen. It had already been known that, in general, the presence of hydrogen on the surface of metals weakens the adsorption of alkenes and favors pi adsorption rather than the di-sigma bonding that dominates on clean surfaces [H. Ofner, F. Zaera, J. Phys. Chem. 101 (1997) 396: F. Zaera, D. Chrysostomou, Surf. Sci. 457 (2000) 89]. However, here we learned that, at least on Pt(111), this effect is stronger with cis than with trans alkenes. TPD experiments with 1,4-difluoro-2-butenes, where fluorine substitutions were used to label and differentiate between the cis and trans isomers, showed that the trans isomer is indeed the more stable of the two on the clean (hydrogen-free) Pt(111) [I. Lee, M. K. Nguyen, T. H. Morton, F. Zaera, J. Phys. Chem. C 112 (2008) 14117]. Direct studies with 2-butyl surface intermediates, prepared by activation of 2-halobutanes, also indicated the preferential production of trans-2-butene [I. Lee, F. Zaera, J. Phys. Chem. C 111 (2007) 10062]. On the other hand, quantum mechanics (DFT) calculations clearly attested to both a switch from di-sigma adsorption on clean Pt(111) to pi bonding on hydrogen-saturated surfaces, and, more revealing, the reverse of relative stability from the trans to the cis isomer of the alkene [F. Delbecq, F. Zaera, J. Am. Chem. Soc. 130 (2008) 14924].
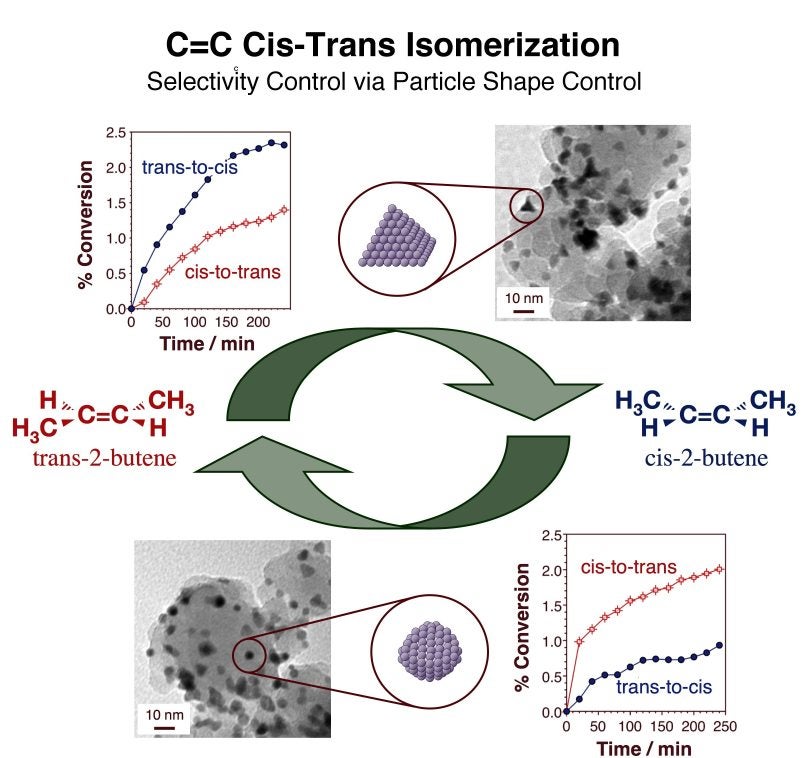
The DFT study also pointed to the role that the structure of the surface plays in determining the bonding strength of alkenes on metals, which appears to be due to, at least in part, a significant reconstruction of the metal surface atoms induced by the adsorption. It was hypothesized that such reconstruction may be driven by the need to minimize the steric interactions between the surface and the end groups of the alkene (which are expected to be more severe with the trans isomer), and that perhaps more open surfaces would require a lesser degree of reconstruction and therefore show the expected higher stability for the trans isomer. This was indeed corroborated experimentally, first by TPD using single-crystal surfaces with more open structures, and later by using supported catalysts consisting of tetrahedral platinum particles, which only expose the (111) planes that promote cis alkene formation [I. Lee, F. Delbecq, R. Morales, M. A. Albiter, F. Zaera, Nature Mater. 8 (2009) 132; I. Lee, R. Morales, M. A. Albiter, F. Zaera, Proc. Nat. Acad. Sci. 105 (2008) 15241]. In the latter, kinetic measurements indicated that the tetrahedral Pt catalysts promote trans-to-cis isomerizations at a rate almost twice as high as they do similar trans-to-cis conversions, and also that the more rounded particles obtained by annealing to high temperatures show a preference for the reverse cis-to-trans reaction (see Figure below). Here is a clear example where stereoselectivity in dehydrogenation steps involving hydrocarbon surface intermediates can be controlled by controlling the structure of the surface of the catalysts used.
Kinetic catalytic data and transmission electron microscopy (TEM) images to indicate the correlation that exists between the structure of the surface of a platinum-based catalysts and its selectivity in alkene cis-trans isomerization conversions. Catalysts consisting of tetrahedral platinum particles, which only expose (111) facets, preferentially promote the formation of the cis isomer (top). Other more round structures, conversely, display a reversed selectivity towards the trans isomer (bottom)