Others
Besides the ongoing projects described on other pages, we have during our tenure at UCR pursued a few additional directions of research in our laboratory in connection with problems in catalysis and materials science.
- Catalysts for the Oxidation of Alcohols
- Hydrocarbon Catalytic Oxidations
- Other Hydrocarbon Conversions using Molecular Beams
- IR Characterization of Supported Catalysts
- Projects in Material Sciences
- Synchrotron-Based Spectroscopies
- Monte Carlo Simulations
Catalysts for the Oxidation of Alcohols
In the past we have carried some catalytic kinetic work on the oxidation of hydrocarbons [F. Zaera, N. R. Gleason, B. Klingenberg, A. H. A. Ali, J. Mol. Catal.146 (1999) 13; F. Zaera, Catal. Today 81 (2003) 149]. Specifically, the oxidation of alcohols on nickel wires and foils was investigated by using a micro-batch reactor [Ali H. Ali and F. Zaera, J. Mol. Catal. A 177 (2002) 215]. We have shown that partial oxidation products can be produced in significant quantities under the right conditions. For instance, the reaction of 2-propanol with oxygen on nickel wires produced acetone almost quantitatively as long as both the oxygen-to-hydrocarbon and the reaction temperature are kept reasonably low. Primary alcohols react in a somewhat less selective way, producing a small amount of the corresponding acid as well as larger quantities of CO and CO2, but significant yields of aldehydes are still possible under the right conditions. The active surface in all these reactions appears to be the thin nickel oxide film that forms immediately upon exposure of nickel metal to the reaction mixture.
|
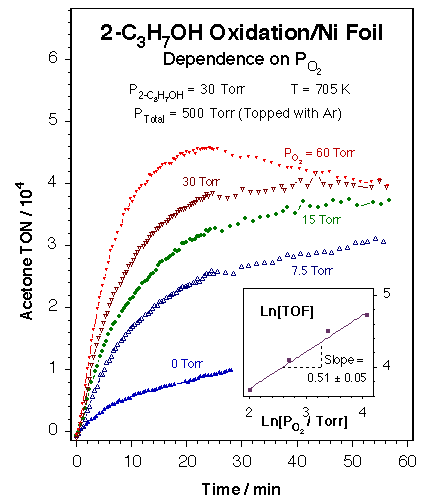
Typical kinetic data for the partial oxidation of alcohols by nickel catalysts. The data in this figure show the dependence of the rate of oxidation of 2-propanol on the partial pressure of oxygen over a Ni foil at 705 ± 2 K. The main panel displays the raw data measured in a batch reactor, in the form of the accumulation of acetone (in turnover numbers, TON = molecules/Ni surface atom·s) as a function of reaction time. The inset provides a Ln - Ln plot of the initial rates of formation of acetone (in turnover frequencies, TOF = TON/time) versus oxygen pressure, as calculated from the raw kinetic data. The order of the reaction with respect to oxygen was estimated to be 0.51 ± 0.05. These experiments prove that the partial dehydrogenation of alcohols to aldehydes or ketones can be accomplished with good selectivity by using nickel foils and oxygen-rich mixtures. Similar results were obtained on silica-supported nickel catalysts, but significant dehydration was seen on alumina-based solids because of the acidity of that oxide. |
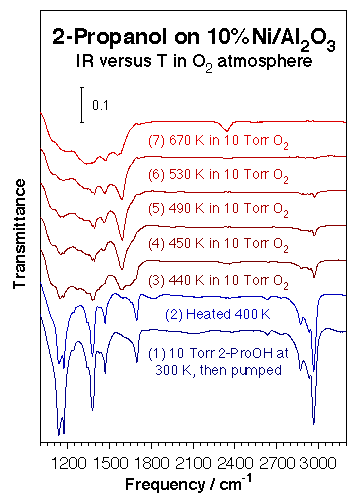
Additional kinetic and infrared characterization experiments have been carried out on supported nickel catalysts. The kinetic behavior seen on nickel foils was by and large reproduced qualitatively on supported nickel catalysts. Indeed, nickel particles deposited on neutral silica powders display similar high selectivities towards alcohol dehydrogenation to aldehydes or ketones. When alumina is used instead, however, significant dehydration is seen as well. For instance, when using a 2-propanol:oxygen 2:1 mixture, the 70% selectivity for 2-propanol dehydrogenation to acetone seen on a nickel foil goes down to only about 30% on Ni/alumina. Additional transmission infrared (IR) spectroscopy characterization studies allow for the identification of most of the intermediates in these reactions. The figure below shows a typical set of spectra for the thermal chemistry of 2-propanol on a 10% nickel/alumina catalyst in the presence of gas-phase oxygen. In this particular instance, it is clear that the original alcohol decomposes to acetone between 440 and 450 K, but that some acetate surface species are also produced under certain conditions. In fact, in the case of ethanol on Ni/SiO2, ethoxide, acetaldehyde, acetate, formate and CO2 were all sequentially identified with increasing temperature. Finally, again, a thin and partially oxidized layer (as identified by CO infrared probing experiments) seems to optimize the partial oxidation.
|
Transmission infrared absorption spectra obtained during the thermal oxidation of 2-propanol on 10% Ni/Al2O3 after sequential exposures to 10 Torr of the alcohol and 10 Torr of oxygen. A change in the nature of the adsorbed species is seen about 440 K, from molecular adsorption to metal-adsorbed acetone, with its characteristic IR bands around 1378, 1472, and 1590 cm-1 (the symmetric and asymmetric methyl deformations and the carbonyl stretching modes, respectively). Experiments such as these allow for the identification of potential surface intermediates during catalysis.
|
Hydrocarbon Catalytic Oxidations
Some years ago we looked into the kinetics of alkane oxidations using transition metal surfaces [M. Aryafar and F. Zaera, Catal. Lett. 48 (1997) 173]. Specifically, the kinetics of the total oxidation of alkanes (methane, ethane, propane, n-butane and isobutane) over Ni, Pd, and Pt foils was studied under fuel-lean conditions by using a recirculating batch reactor with mass spectrometry detection. Approximate first-and zeroth-order kinetics with respect to the hydrocarbon and oxygen concentrations, respectively, was observed in all cases. Significantly slower rates were seen for methane conversion, but other subtle changes were also identified among the other hydro-carbons as well. The reactivity trends could be clearly correlated with C-H bond strengths, with higher rates seen for the longer and more branched hydrocarbon chains. Also, platinum was found to be the most active catalyst for the oxidation of all the alkanes except methane, which is oxidized faster on the nickel substrate. The variations in activity among the three catalysts were shown to be associated mostly with changes in the pre-exponential factor, not the activation energy, suggesting that they have to do with the surface density of active sites on the surface. The nature of the active catalyst during reaction was determined by simple inspection to be the metallic phase in the cases of Pt and Ni but an oxide layer in the case of Pd.
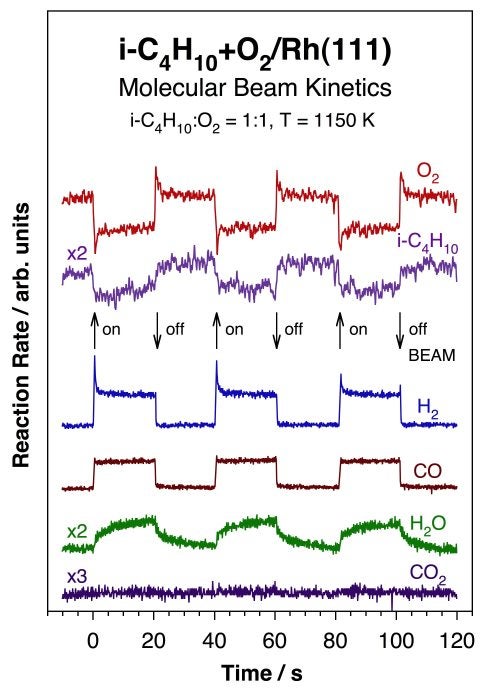
More recently, we have been investigating the kinetics of high-temperature alkane oxidations to produce hydrogen [J. Wilson, H. Guo, R. Morales, E. Podgornov, I. Lee and F. Zaera, Phys. Chem. Chem. Phys. 9 (2007) 3830; J. N. Wilson, R. A. Pedigo, and F. Zaera, J. Am. Chem. Soc. 130 (2008) 15796]. The Figure below illustrates the type of data obtained from these studies, in this case for the steady-state catalytic conversion of isobutane with oxygen on a Rh(111) single-crystal surface held at a temperature of 1150 K. Shown are the traces obtained using molecular beams for the rates of oxygen and isobutane consumption as well as those for the production of molecular hydrogen, carbon monoxide, carbon dioxide, and water. The beam was interrupted periodically to get better estimates of the rate differentials corresponding to the conversion of the reaction mixture from the beam impinging directly on the surface. Several observations are worth highlighting from these data: (1) steady-state alkane conversion can be clearly reached for this system; (2) the stoichiometry measured from the data suggests the following as the primary reaction: i-C4H10 + 2 O2 --> 4 CO + 5 H2; (3) a competing minority channel is also indicated by the production of a small amount of water. However, the absence of any CO2 production argues against the expected water-shift reaction; (4) the slow transient measured for the formation of water highlights the secondary nature of the surface reaction responsible for its production; and (5) the mirroring spikes in the O2 and H2 traces seen immediately after blocking and unblocking of the beam point to the formation of other transient species on the surface.
Isothermal kinetic data for the conversion of a 1:1 isobutane+O2 mixture on a Rh(111) single-crystal surface at 1150 K, acquired by using an effusive molecular beam in a setup similar to that used for sticking coefficient measurements. Here, the evolution of the partial pressures of the reactants (oxygen and isobutane) and products (hydrogen, carbon monoxide, water, and carbon dioxide) are shown as a function of time under steady-state catalytic conditions. The impinging gas beam was interrupted periodically, as indicated by the arrows in the plot, to directly determine the reaction rates over the background signals. These data helped identify the products that form in this system (H2, CO, and H2O, but not CO2), and provided, after appropriate calibration, absolute reaction rates and stoichiometries. Additional information was afforded by the transient behavior that takes place immediately after blocking and unblocking of the beam. Note in particular the slow evolution of the water signal, an observation that suggests a secondary reaction on the surface.Other Hydrocarbon Conversions using Molecular Beams
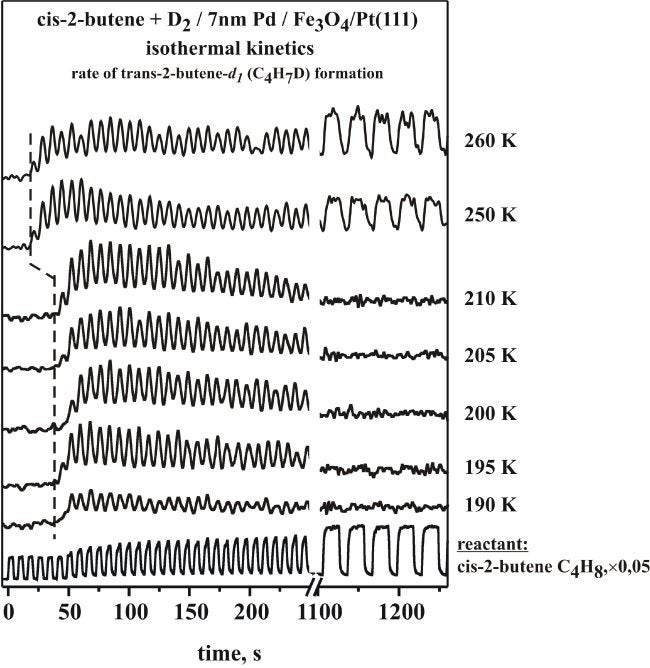
We have long been interested in emulating the kinetics of catalytic reactions under controlled conditions. This can be sometimes achieved by using molecular beams. In addition, it is important to also reproduce closely the elements of real catalysts in the model systems used for UHV studies. In a collaboration with Prof. Freund at the Fritz-Haber Institute in Berlin, we have used a model system comprised of Pd particles evaporated on an oxide flat surface to investigate the catalytic conversion of olefins, with particular emphasis on understanding cis-trans isomerizations [B. Brandt, J.-H. Fischer, W. Ludwig, J. Libuda, F. Zaera, S. Schauermann, H.-J. Freund, J. Phys. Chem. C 112 (2008) 11408; B. Brandt, W. Ludwig, J.-H. Fischer, J. Libuda, F. Zaera, S. Schauermann, J. Catal. 265 (2009) 191]. It was found that selectivity towards cis-trans isomerization and hydrogenation depends critically on the nature of the carbonaceous deposits, which are typically present during reaction on real catalysts. At low temperatures (190 - 210 K) both reaction pathways were found to proceed on the initially clean surface, but the catalytic activity was observed to quickly vanish, presumably because of the accumulation of hydrocarbon species on the surface. At temperatures above 250 K, on the other hand, a sustained catalytic activity towards cis-trans isomerization was observed over long periods of time. The transition among these types of behavior are illustrated by the data in the Figure below, taken by using periodic pulses of the olefin while keeping the D2 beam continuously on. Interestingly, no catalytic activity could be sustained for the competing hydrogenation on the initially clean catalyst even at these temperatures. Only when highly dehydrogenated carbonaceous fragments were preadsorbed on the surface was it possible to induce a persisting catalytic activity for the hydrogenation (and also the isomerization) of the alkene on our supported palladium particles. Possible reasons of this unique vacuum catalytic behavior are discussed, including different spatial requirements for the competing reaction pathways and changes in the adsorption state of deuterium on and beneath the surface modified by the carbonaceous deposits.
Results from molecular beam experiments carried out on Pd/Fe3O4 at different temperatures in the range from 190 to 260 K. Shown is the time evolution of the product of cis-trans isomerization (trans-2-butene-d1) for each temperature, together with that of the reactant (cis-2-butene, bottom trace). Below 210 K the reaction rates are high at the beginning but return to zero after prolonged exposures. Above 250 K, on the other hand, there is a shorter induction period, and sustained catalytic activity towards isomerization is possible.
IR Characterization of Supported Catalysts
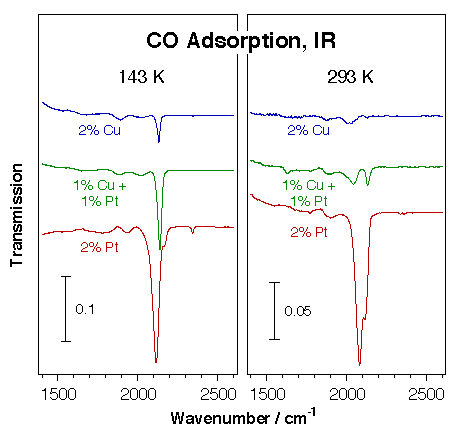
For one, we use infrared absorption spectroscopy extensively to characterize the surface chemistry of the active phase of supported catalysts. In one example, the possibility of alloying platinum catalysts with copper was explored as a way to tune their selectivity towards less dehydrogenation. The composition of several supported Pt-Cu catalysts was characterized by infrared spectroscopy of adsorbed carbon monoxide. The figure below displays representative data from those experiments. They clearly show the preferential segregation of the copper to the surface: the 50% Pt:50% Cu catalyst was proven to behave very similarly to the copper-only sample. Subtle effects were nevertheless seen during the oxidation and reduction of the different solids.
|
Transmission infrared spectra for carbon monoxide adsorbed on Pt-Cu mixed-metal particles dispersed on a high surface area silica support. Three samples were studied, all at the same 2% loading: pure copper, pure platinum, and a 1:1 Pt:Cu mixture. It is seen here that the alloy behaves in a similar way as the pure copper catalyst, a fact indicated by the weak nature of the CO adsorption (most of its IR signal disappears upon warming up to room temperature), and by the high frequency of the C–O bond (about 2130 cm-1). This provides a clear indication for the preferential segregation of copper to the surface. |
Our capabilities for catalyst characterization have brought many collaborators to our laboratory. Recent examples of these projects include a group from the Advanced Materials Research Center in Chihuahua, Mexico. They spent some time in our lab looking into the nature of their nickel-cobalt-molybdenum disulfide catalysts for hydrodesulfurization [M. A. Albiter, R. Huirache-Acuña, F. Paraguay-Delgado, F. Zaera, and G. Alonso-Núñez, J. Nanosci. Nanotechnol. 8 (2008) 6437]. A group from the Federal University of Lavras in Brazil also visited us recently to further their understanding of the performance of Nb-doped hematite catalysts for the decomposition of isopropanol [L. C.A. Oliveira, F. Zaera, I. Lee, D. Q. Lima, T. C. Ramalho, A. C. Silva and E. M.B. Fonseca, Appl. Catal. A 368 (2009) 17]. A group from the Central University of Venezuela brought their PtSn/H[Al]ZSM5 Catalysts for characterization [R. Morales, L. Melo, A. Llanos and F. Zaera, J. Mol. Catal. A 228 (2005)]. A visitor from the La Salle University in the Philippines spent time at UCR under a Fulbright Research Scholarship to characterize her metal oxide/activated carbon catalysts for VOC removal.
Projects in Material Sciences
We have also worked on several problems related to issues in materials chemistry. In one example, XPS investigations were carried out on the reactivity of aluminum surfaces with high-pressure gases in connection with gas storage tanks. The inside surface of several aluminum gas cylinders were analyzed after different treatments. Those experiments were successful in determining the source of staining, which was traced back to the use of excessive lubricant in some parts during the extrusion of the cylinders, and lead us to propose the implementation of some extra acid washing at the end of the manufacturing process, since the previous procedure proved insufficient on the lubricant and too much on the rest of the cylinder. This project was carried out in conjunction with people at the Riverside factory of the Luxfer Group.
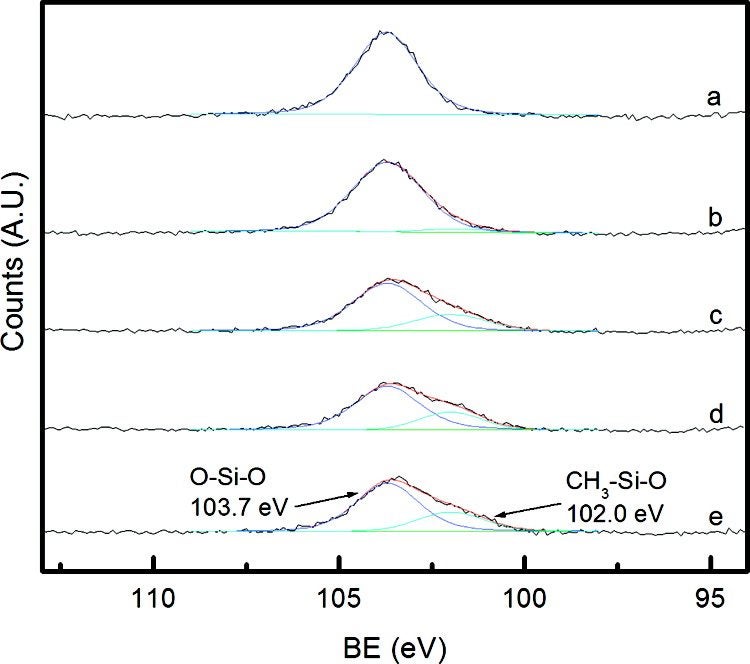
In another, more recent example, we have collaborated with the group of Prof. Yushan Yan at the Department of Chemical Engineering of UCR to characterize a number of Hydrofluoric Acid-Resistant and Hydrophobic Pure-Silica-Zeolite MEL Low-Dielectric Constant Films as a function of the preparation procedure [C. M. Lew, Y. Liu, B. Day, G. M. Kloster, H. Tiznado, M. Sun, F. Zaera, J. Wang, and Y. Yan, Langmuir 25 (2009) 5039]. A new technique for the silylation of pure-silica-zeolite MEL low-k films was developed in which the spin-on films are calcined directly in trimethylchlorosilane or 1,1,1,3,3,3-hexamethyldisilazane (HMDS) in order to protect the films against corrosive wet etch chemicals and ambient moisture adsorption. In an alternative procedure, HMDS is also added to the zeolite suspension before film preparation. Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, water-soak tests, and HF etch tests were performed to characterize the films. The Figure below shows some of the resulting XPS data.
XPS Si 2p traces from zeolite films after different treatments. The larger peak at 103.7 eV results from Si bonded to the oxygens in SiO2, while a secondary peak that emerges at a lower binding energy of 102.0 eV corresponds to the Si coordinated with oxygen and a methyl group. The ratio of the O-Si-CH3 peak at 102.0 eV to the O-Si-O peak at 103.7 eV indicates the relative amounts of methylation. It can be seen from these data that the PSZ (as spun, a) MEL film does not exhibit any methyl group functionalization, and that the traditionally-silylated films (b) show a slight peak at 102.0 eV. In contrast, the larger contributions at 102.0 eV for the other traces indicate a higher amount of methyl functionalization in the TMCS (calcined in a trimethylchlorosilane environment, c), HMDS (calcined in a 1,1,1,3,3,3-hexamethyldisilazane environment, d), and presilylated (e) films. Furthermore, the ratio in the presilylated film is the largest at 0.35. This result is understandable, since the presilylation process exposes the nanoparticle suspension to more silylation agent than the TMCS, HMDS, and traditionally-silylated films.
Synchrotron-Based Spectroscopies
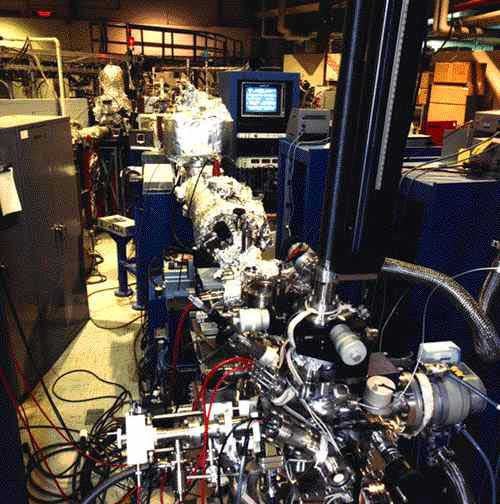
Our research has also lead us to develop and explore new surface sensitive techniques. For instance, we have in the past relied on the use of synchrotron-based near-edge X-ray photoelectron (NEXAFS) and angle-resolved ultraviolet photoelectron spectroscopy (ARUPS) spectroscopies for the determination of electronic structures and adsorption geometries [F. Zaera, E. Kollin and J. L. Gland, Chem. Phys. Lett., 121 (1985) 464; D. W. Moon, S. Cameron, F. Zaera, W. Eberhardt, R. Carr, S. L. Bernasek, J. L. Gland and D. J. Dwyer, Surf. Sci. 180 (1987) L123; F. Zaera, D. A. Fischer, R. G. Carr and J. L. Gland, J. Chem. Phys. 89, (1988) 5335; J. L. Gland, F. Zaera, D. A. Fischer, R. G. Carr and E. B. Kollin, Chem. Phys. Lett. 151 (1988) 227; G. Bradael, W. T. Tysoe and F. Zaera, Langmuir 5 (1989) 899; J. P. Fulmer, W. T. Tysoe and F. Zaera, Langmuir 6 (1990) 1229; L. P. Wang, W. T. Tysoe, R. M. Ormerod, R. M. Lambert, H. Hoffmann and F. Zaera, J. Phys. Chem. 94 (1990) 4236; H. Hoffmann, F. Zaera, R. M. Ormerod, R. M. Lambert, L. P. Wang and T. W. Tysoe, Surf. Sci. 232 (1990) 259; H. Hoffmann, F. Zaera, R. M. Ormerod, R. M. Lambert, J. M. Yao, D. K. Saldin, L. P. Wang, D. W. Bennett and T. W. Tysoe, Surf. Sci. 268 (1992) 1; R. M. Ormerod, R. M. Lambert, H. Hoffmann, F. Zaera, J. M. Yao, D. K. Saladin, L. P. Wang, D. W. Bennett and W. T. Tysoe, Surf. Sci. 295 (1993) 277; F. Zaera, in X-Ray Absorption Fine Structure for Catalysis and Surfaces, Y. Iwasawa, ed., World Scientific, Singapore, 1996, pp. 362-371]. These experiments are carried out at a synchrotron facility because of the need of a tunable light source. A particularly interesting extension of the use of NEXAFS to the characterization of surface adsorbates involved the design of a fluorescence-detection scheme for the detection of low-Z elements in experiments under non-vacuum conditions. An original detector was designed and optimized for sulfur detection [J. Stöhr, E. B. Kollin, D. A. Fischer, J. B. Hastings, F. Zaera and F. Sette, Phys. Rev. Lett. 55 (1985) 1468; D. A. Fischer, J. B. Hastings, F. Zaera, J. Stöhr and F. Sette, Nucl. Instr. Methods Phys. Res. A246 (1986) 561)], and further developed to extend its use to carbon, nitrogen, and oxygen [D. A. Fischer, F. Zaera and J. L. Gland, J. Physique 48 (1987) C9-1097; J. L. Gland, F. Zaera, D. A. Fischer and S. Shen, Catalysis 1987, J. W. Ward Ed., Elsevier, Amsterdam 1988, pp. 835-843]. A picture of the beam line used for the C-fluorescence instrument is shown below. This technique has been used to determine the kinetics of displacement of CO by other gases from transition metal surfaces [F. Zaera, D. A. Fischer, S. Shen and J. L. Gland, Surf. Sci. 194 (1988) 205; S. Shen, F. Zaera, D. A. Fischer and J. L. Gland, J. Chem. Phys. 89 (1988) 590; J. L. Gland, S. Shen, F. Zaera and D. A. Fischer, J. Vac. Sci. Technol. A6 (1988) 2426; J. L. Gland, D. A. Fischer, S. Shen and F. Zaera, J. Am. Chem. Soc. 112 (1990) 5695].
|
Picture of the beam line equipped with our fluorescence detection NEXAFS apparatus. This system, installed at the National Synchrotron Light Source in the Brookhaven National Laboratory, is capable of detecting C, N, and O atoms of adsorbates on single-crystal surfaces under atmospheric pressures. |
Monte Carlo Simulations
One of our long-term goals has been to understand the kinetics of reactions on solid surfaces. To this end, our experimental measurements have been sometimes complemented with Monte Carlo computer simulation [F. Zaera and I. Rusinek, J. Comp. Chem. 2 (1981) 402; T. Nordmeyer and F. Zaera, J. Chem. Phys. 97 (1992) 9345]. One of our first studies in this direction was focused on the non-dissociative adsorption of gas-phase molecules onto a spatially homogeneous square lattice using a method which accounts for the existence of an extrinsic precursor state [J. Phys. Chem. Solids, 3, 95, 1957].
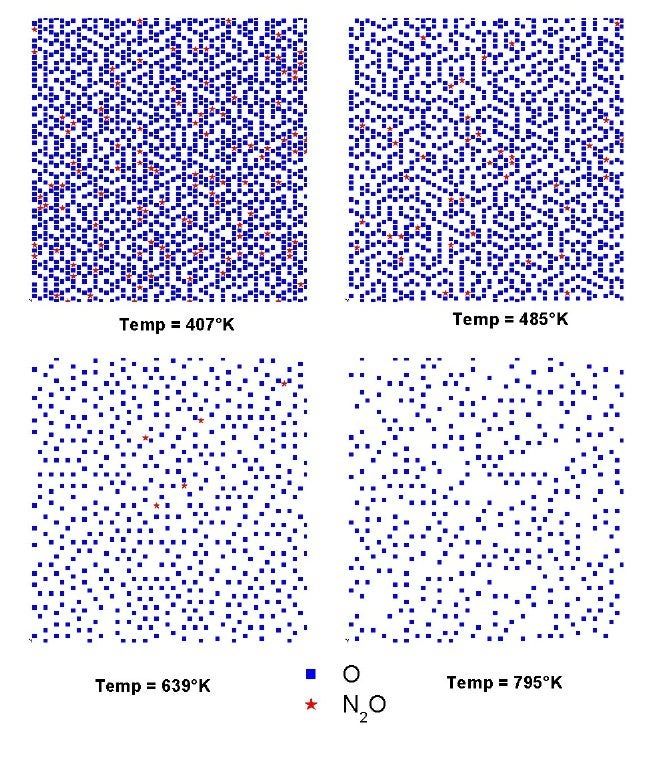
We have also been working with Professor Giorgio Zgrablich to simulate the kinetics of NO reduction and molecular nitrogen formation on Rh(111) surfaces [V. Bustos, C. S. Gopinath, R. Unac, F. Zaera and G. Zgrablich, J. Chem. Phys. 114 (2001) 10927; F. Zaera, S. Wehner, C. S. Gopinath, J. L. Sales, V. Gargiulo and G. Zgrablich, J. Phys. Chem. B 105 (2001) 7771;V. Bustos, R. Unac, F. Zaera and G. Zgrablich, J. Chem. Phys. 118 (2003) 9372; L. A. Avalos, V. Bustos, R. Uñac, F. Zaera and G. Zgrablich, J. Mol. Catal. A 228 (2005) 89; L. A. Avalos, V. Bustos, R. Unac, F. Zaera, G. Zgrablich, J. Phys. Chem. B 110 (2006) 24964; R. O. Unac, V. Bustos, J. Wilson, G. Zgrablich, and F. Zaera, J. Chem. Phys. 125 (2006) 074705; F. Zaera, J. L. Sales, M. V. Gargiulo, M. Ciacera, and G. Zgrablich, J. Phys. Chem. C 111 (2007) 7795]. These Monte Carlo simulations have proven particularly suited to the study of inhomogeneous systems. One good example of this is the case of the nitrogen islands that appear to form on Rh(111) during the catalytic reduction of nitrogen oxide. Our Monte Carlo simulations were used to explain the isotopic distributions observed by our molecular beam experiments in terms of the formation of islands with the nitrogen isotopes distributed in an "onion" structure, the 14N atoms in a core surrounded by a 15N outer shell. Appropriate kinetic parameters were extracted from those calculations. In another example, the Figure below shows how snapshots of the surface during the conversion of N2O on Rh(111) can be used to determine the potential ordering of adsorbates on the surface.
Surface snapshots from the Monte Carlo simulations at the end of the N2O conversion on Rh(111). Pictures of the spatial distributions of the adsorbed oxygen and N2O are shown here for four different temperatures. Random distributions, without island formation, were obtained in all cases, but significantly higher oxygen coverages were obtained at lower temperatures.
More recently, we are also looking into the physical details behind the chiral templating of metal surfaces. This work is in its preliminary stages at present.