ALD
- Introduction
- ALD of Metal Nitrides
- Surface Chemistry of Organometallic Precursors
- ALD of Copper Films
- CVD and ALD Using Metal Carbonyls
Introduction
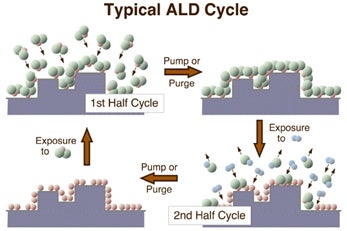
The main objective of this work is to develop a molecular-level knowledge of the reactions associated with atomic layer deposition (ALD). ALD relies on the alternate use of two or more self-limiting and complementary reactions to deposit materials on solid surfaces one monolayer at a time. The figure below depicts schematically how this works. ALD techniques have been recently advanced as a powerful way to deposit thin films in a controlled manner for the microelectronic industry. There are, however, a number of still unresolved problems involving the stoichiometry, structure, and chemical quality of the deposited films. We are working to shed some light on those issues by advancing the understanding of the mechanism of the underlying surface reactions [F. Zaera, J. Mater. Chem. 18 (2008) 3521].
|
Schematic representation of atomic layer deposition (ALD) processes, which are based on two self-limiting and complementary reactions. By alternating the two chemical steps, films can be grown conformally and with atomic thickness resolution even on rough surfaces. However, several fundamental issues such as the elimination of impurities and the control of film morphology need to be resolved before ALD can be widely used in microelectronics fabrication. The aim of our work in this project is to address those problems from a mechanistic point of view. |
ALD of Metal Nitrides
Our efforts in this area were started by investigating the surface chemistry of the deposition of metal nitrides. The case of the growth of TiN films from TiCl4 and NH3 is perhaps the best ALD studied to date, but many questions remain still. For instance, it is not yet clear what the stoichiometry of the resulting films is, how the reduction of the titanium atoms (from Ti4+ in TiCl4 to Ti3+ in TiN) occurs, or what the oxidation byproduct is. Moreover, although TiN films grown by ALD usually contain oxygen and other contaminants, it has also not yet been clearly established what the sources of those contaminants are, or how they get incorporated into the film. Not even the specific composition and stoichiometry of the deposited film has been properly determined. We have initiated our research on ALD processes by focusing on the surface chemistry of this type of metal nitride films growth [H. Tiznado, F. Zaera, J. Phys. Chem. B. 110 (2006) 13491; M. Xu, H. Tiznado, B.-C. Kang, M. Bouman, I. Lee, F. Zaera, J. Kor. Phys. Soc. 51 (2007) 1063; H. Tiznado, M. Bouman, B.-C. Kang, I. Lee, F. Zaera, J. Mol. Catal. A 281 (2007) 35].
XPS data corroborate most of the basic behavior expected in ALD processes, but also highlights some surprising findings. Specifically, it was determined that the initial exposure of the surface to TiCl4 leads to the deposition of titanium in the +3 oxidation state, and builds a layer of surface species with a final TiCl3.5 stoichiometry. Moreover, the species left after subsequent ammonia dosing are not stoichiometric either, but rich in nitrogen. This is in fact the case all throughout the buildup of thicker films via multiple cycles with alternating exposures to TiCl4 and NH3. All our evidence, including the complementary depth profiling spectra shown below, which indicates the preferential initial removal of Ti4+ species, points to the presence of a Ti3N4 thin layer on top of the TiN film. The overall conclusion is that the reduction of titanium may take place during the TiCl4 half-cycle, and not by the ammonia as widely believed. This suggests that the key for improving that ALD process may be to find a Ti precursor that disproportionates with ease or does not require to be reduced at all.
 |
Summary of the XPS data obtained in studies of the ALD of titanium nitride films using TiCl4 and ammonia. The data correspond to the W (the substrate), N, and Ti average atomic surface concentrations and the N/Ti atomic ratio obtained as a function of the number of ALD cycles during film growth (left) and of the sputtering time once a thick film had been grown (right). N/Ti ratios higher than unity were always observed during growth but not after light sputtering, indicating the formation of a thin Ti3N4 layer on top of the growing TiN film. The results suggest that the reduction of the titanium ions occurs via disproportionation of the TiCl4 precursor, not by reaction with ammonia as commonly thought. |
This study also shed some light on the origin of the chlorine and oxygen contaminants typical in metal nitride deposition using chlorides. As mentioned above, the reason for the chlorine incorporation in the growing films could be traced to readsorption of the HCl byproduct that forms during exposures to ammonia. This effect could be minimized by extending the purging periods or by performing additional treatments with ammonia, a procedure that we suggest may be successful in minimizing halide incorporation during metal oxides film growth using metal halides. It is also worth pointing out that, during our TiN ALD studies, we briefly explored the possibility of growing TiO2 films using TiCl4. It was shown that, indeed, it is possible to deposit nice oxide films using water as the second reactant, at rates about half those seen for TiN deposition (~0.3 Å/cycle). Molecular oxygen, on the other hand, proved quite inefficient for this purpose, displaying growth rates almost an order of magnitude lower. The films obtained with both reactants were stoichiometric and with all titanium atoms in the Ti4+ oxidation state, indicating complete oxidation as desired.
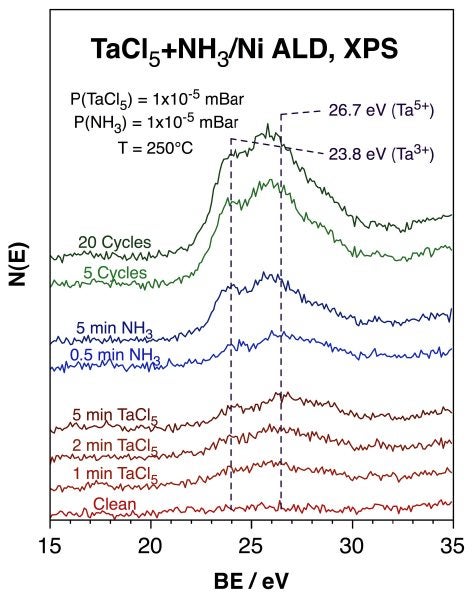
Similar conclusions were reached for the ALD processes of TaN films using TaCl5 and NH3. TaN films are perhaps some of the most promising for use as diffusion barriers in the microelectronics industry. For instance, analogous behavior is seen in the Ta 4f XPS data shown below. The bottom four traces, which correspond to the initial uptake of TaCl5 on a nickel foil at 250°C, show how the first molecules to adsorb on the substrate are reduced to a Ta3+ state. A second pair of XPS peaks does grow after larger exposures to TaCl5 with the binding energies expected for TaCl5. Subsequent exposure to ammonia (next two traces) shifts most of the TaCl5 XPS signal back to the Ta3+ state, as the intensity of the N 1s XPS feature also grows and that of Cl 2p XPS peak is almost completely suppressed; all trends expected for TaN formation. Finally, repetition of this cycle leads to the slow growth of a TaN film (top two traces).
|
Ta 4f XPS for the growth of TaN films on a nickel foil at 250°C using TaCl5 and ammonia. The reactants were dosed using pressures of approximately 1 x 10-5 mBar. The bottom four traces correspond to the initial uptake of TaCl5, and point to the partial reduction of the metal to Ta3+ upon adsorption, as in the TiN case. Further exposure to ammonia (next two traces) drives the reduction further, presumably because of the formation of the TaN film. The top two traces show how thicker films can be grown by repeating this cycle. The final average stoichiometry of that film is approximately TaN, but an oxide species is also manifested by additional Ta 4f XPS intensity around 25.8 eV. |
Another issue the ALD of TaN shares with that of TiN is that of the incorporation of impurities in the film, in particular the codeposition of a metal oxide. In this case the problem is evidenced by the excess intensity in the Ta 4f XPS signal at 26.7 eV beyond what is expected for the Ta 4f5/2 peak of the Ta3+ species, and also by the O 1s XPS feature seen at approximately 531.8 eV (data not shown). The stoichiometry of this oxide estimated from the areas of the 26.7 eV Ta 4f7/2 and 531.8 eV O 1s XPS peaks in the final film is Ta2O5, and the binding energies seen for the two elements are consistent with this interpretation. The oxide film appears to develop at the interface between the nickel substrate and the TaN growing film, and it is removed last in subsequent depth profiling experiments by argon sputtering.
Surface Chemistry of Organometallic Precursors
The current trend in metal nitride ALD is to move away from metal halides and to use organometallic precursors instead. With that in mind, we have been investigating the surface chemistry of a series of tetraethylmethylamido precursors. In the case of the deposition of zirconium nitride using Zr[N(C2H5)(CH3)]4 (TEMAZ), our XPS data again point to a reduction of the Zr metal upon adsorption. Two more key observations derived from this study: (1) there seems to be an induction period before the deposition of the zirconium starts; and (2) an oxide is formed here too. In this system the oxide appears to block further film growth, since no increase in Zr 3d or N 1s XPS intensity is seen after 20 ALD cycles.
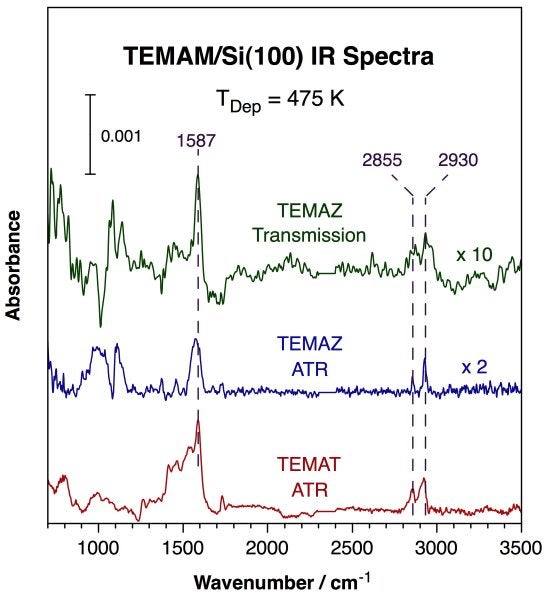
Additional experiments were carried out in a cell capable of in-situ infrared surface characterization in order to follow the thermal chemistry of the amine ligands in the organometallic precursors during the uptake. Infrared absorption spectroscopy data such as those shown below point to partial decomposition of those compounds on silica or silicon surfaces upon adsorption. On the one hand, most of the peaks around 2700-3000 cm-1 due to C-H stretching vibrational modes are preserved, but, on the other, large new peaks around ~ 900, 1600, and 2000 - 2200 cm-1 due to single, double, and triple carbon-nitrogen bonds, respectively, appear. The modes for the N-CH3 moiety seem to be all gone, suggesting preferential conversion at that position, but both nitrile and isonitrile species appear to form, which means that some amine groups may detach from the metal center before dehydrogenating. Two more observations derive from the data with TEMAZ: (1) an induction period is seen for the TEMAZ deposition, the same as in the XPS experiments; and (2) thick ZrN films can be grown with TEMAZ alone, presumably by a regular CVD process, as manifested by a golden finish obtain on the powder (and the silicon wafer) after extended exposures. One particularly important observation from our IR work with the titanium counterpart (TEMAT) is that a beta-H elimination step takes place preferentially at the ethyl moiety of these precursors [B.-C. Kan, J.-H. Boo, I. Lee, and F. Zaera, J. Phys. Chem. A, 113 (2009) 3946]. This conclusion refutes previous reports, and speaks to the relative stability of different metal amido precursors.
|
Infrared absorption spectra from Si(100) surfaces after saturation with TEMAM at 475 K. Three traces are shown, for spectra of TEMAT and TEMAZ recorded using the ATR IR setup (bottom two traces), and for data of TEMAZ taken with the transmission IR apparatus (top trace). All these spectra share similar prominent peaks at 1587, 2855 and 2930 cm-1, all associated with a surface imine. These data attest to both the feasibility of using our IR setups for the proposed ALD studies and the complexity of the chemistry involved. Several surface species may form during thermal activation of ALD precursors, and their characterization may shed some light on the mechanism by which the films are grown. |
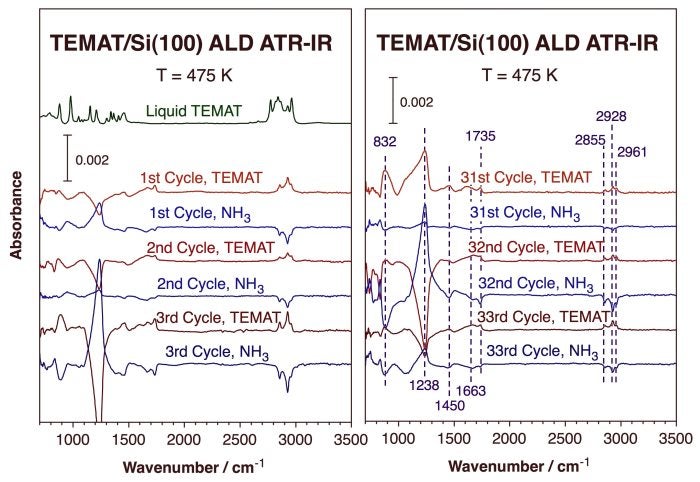
In terms of full ALD processes, the Figure below displays the IR absorption traces obtained after selected half-cycles during the growth of TiN films using TEMAT and ammonia. Several observations become apparent: (1) the alternate formation and disappearance of the N-methylethylidenimine species identified by the experiments described above upon exposures to TEMAT and ammonia, respectively, is evidenced by the alternate growth and removal of the peaks at 1450, 1663, 1735, 2855, 2928, and 2961 cm-1; (2) the absence of any signals for N-H stretching vibrational modes, an indication of the lack of buildup of any NHx species on the surface; (3) the appearance and disappearance of the peak at 832 cm-1 upon exposures to TEMAT and NH3, respectively, reflecting the formation of Ti-N bonds; and (4) the large signal intensities swings observed at 1238 cm-1 due to the optical phonon modes of amorphous silicon oxide up to 30+ ALD cycles, indicating that some silicon substrate remains exposed still after so many ALD cycles.
ATR infrared adsorption spectra of a Si(100) surface after each half-cycle during ALD deposition at 475 K using TEMAT and ammonia, respectively. Each trace has been ratioed against the one obtained right after the previous half cycle in order to highlight the new changes: positive peaks represent the formation of new species whereas negative signals indicate the removal of surface moieties. The alternating formation and removal of imine species after TEMAT and NH3 exposures, respectively, is evident even after 30 cycles, as is the presence of some exposed silicon oxide. The spectra of liquid TEMAT is also provided for reference (left, top).ALD of Copper Films
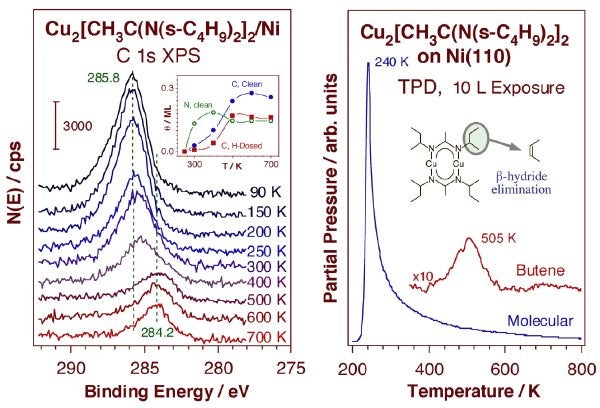
More recently, a few probing studies have been carried out to test the surface chemistry of bis[(N,N'-di-sec-butyl acetamidinate)Cu], a precursor that has shown great promise for the growth of copper films. XPS experiments on both a Ni(110) single crystal and a cobalt polished polycrystalline foil showed clear shifts in the C 1s and N 1s XPS peaks as a function of temperature indicative of surface conversion, and also some copper deposition. Interestingly, at least two steps could be identified by the different onsets in the shifts of the C vs. N XPS data, as shown in the Figure below. TPD data also indicate a beta-H elimination step to produce butene at relatively high temperatures. A temperature window between approximately 350 and 450 K was identified for the clean deposition of the precursor on the surface: lower temperatures are insufficient for activation of the dissociative adsorption, and higher temperatures lead to continuous decomposition beyond Cu monolayer saturation [Q. Ma, H. Guo, R. G. Gordon, and F. Zaera, Chem. Mater. (2009)]. Approximately three ALD cycles are required to reach full Cu monolayer saturation, the equivalent of a film growth rate of approximately 0.75 Å/cycle under pure ALD conditions. Preadsorption of hydrogen on the surface does not modify any of this behavior because of its rapid desorption at temperatures below 350 K once the gas-phase H2 is removed. Accordingly, copper precursors leading to relatively stable organic surface intermediates are required in ALD, because their clean removal can only happen in the second half-cycle of processes that rely on hydrogenation reactions.
Left: XPS data for bis[(N,N'-di-sec-butyl acetamidinate)-Cu] adsorbed on a nickel surface. The main frame displays C 1s XPS spectra as a function of annealing temperature, and shows both a loss of signal above 200 K due to molecular desorption and a shift in the remaining peak because of further surface conversion. The inset summarizes the estimated coverages of the new surface species. Right: Corresponding TPD data, reporting molecular desorption at 240 K and butene production at ~500 K. These data point to the complexity of the surface chemistry involved, which includes several steps, and a high-temperature beta-H elimination.Finally, we have in the past carried out extensive studies on the thermal chemistry of metal carbonyls on nickel and platinum substrates in connection with metal chemical vapor deposition (CVD) processes [F. Zaera, J. Vac. Sci. Technol. A7 (1989) 640; F. Zaera, Langmuir 7 (1991) 1188; F. Zaera, Surf. Sci. 255 (1991) 280; F. Zaera, J. Phys. Chem. 96 (1992) 4609; M. Xu and F. Zaera, Surf. Sci. 315 (1994) 40; M. Xu and F. Zaera, J. Vac. Sci Technol. A14 (1996) 415]. In those studies it was found by using TPD and XPS that all the metal carbonyl precursors investigated (Fe(CO)5, Cr(CO)6, Mo(CO)6, and Co(CO)6) adsorb molecularly at low temperatures, but undergo complete decarbonylation upon heating of the substrate [M. Xu and F. Zaera, J. Vac. Sci Technol. A14 (1996) 415]. It was also observed that for all metal carbonyls except iron the removal of all the CO ligands occurs irreversibly in a narrow range of temperatures. In the case of iron pentacarbonyl, on the other hand, this chemistry was found to take place in a stepwise and reversible manner. Infrared spectroscopy was used to isolate and identify Fe(CO)4 and Fe(CO)3 adsorbed intermediates [F. Zaera, Surf. Sci. 255 (1991) 280], and coadsorption experiments with isotopically labelled 13CO were employed to prove that the decarbonylation could be easily reversed [M. Xu and F. Zaera, Surf. Sci. 315 (1994) 40]. Additional undesirable side reactions were also detected between the adsorbed carbon monoxide and the metal atoms left on the surface which lead to the incorporation of carbon into the growing films as well as to the oxidation of the deposited metal [F. Zaera, J. Phys. Chem. 96 (1992) 4609; M. Xu and F. Zaera, J. Vac. Sci Technol. A14 (1996) 415].
RAIRS data used to identify the intermediates that form during the thermal chemistry of iron pentacarbonyl on metal surfaces. The traces shown here, which correspond to the C–O stretching region of the spectra, provide evidence for an initial isomerization of the Fe(CO)5 precursor upon adsorption into a more reactive C4v geometry, and also for subsequent stepwise decarbonylation steps to produce Fe(CO)4 and Fe(CO)3 surface intermediates at around 185 and 240 K, respectively. Additional TPD experiments using isotopically labeled carbon monoxide were carried out to show that these steps are in fact reversible.
CVD and ALD Using Metal Carbonyls
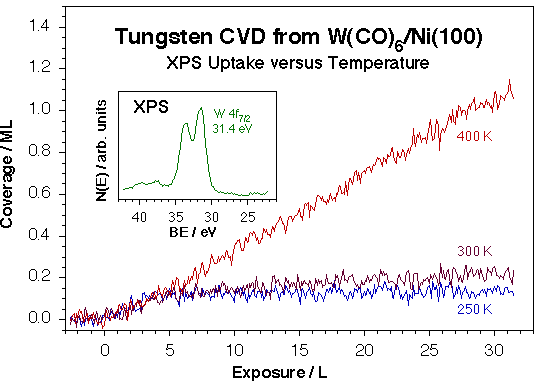
XPS was also employed to follow the kinetics of the film growth in situ during the CVD processes [F. Zaera, Langmuir 7 (1991) 1188; M. Xu and F. Zaera, J. Vac. Sci Technol. A14 (1996) 415]. It was found that the process is self-limiting at low temperatures, and that the sample needs to be kept at temperatures higher than those required for CO desorption to attain steady-state deposition. Representative kinetic data is provided in the Figure below for the case of W(CO)6. Interestingly, the poisoning of the metal carbonyl surface activation by the CO byproduct suggests a procedure for growing metal films with these precursor in a ALD-type manner based on cycles of exposure of the substrate to the M(CO)x gas at low temperatures followed by pumping and annealing to higher temperatures to desorb the adsorbed CO. The initial stages of this procedure were shown to work successfully for the case of W(CO)6 [M. Xu and F. Zaera, J. Vac. Sci Technol. A14 (1996) 415].
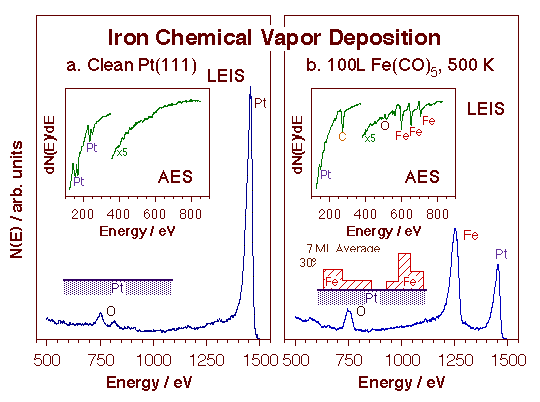
Growth kinetics for the deposition of tungsten films on a nickel surface using W(CO)6. The main frame displays the W coverage uptake as a function of exposure at three different deposition temperatures, as measured by following the W 4f7/2 signal at 31.4 eV after appropriate calibration, while the inset shows the W 4f XPS signature for the resulting tungsten metallic films. These data indicate that the film growth is self-limited at low temperatures, and can be carried out in a linear steady-state fashion only above 300 K. Subsequent XPS, RAIRS and TPD experiments have indicated that the rate-limiting step in this case is the desorption of the carbon monoxide byproduct from the surface.The composition and morphology of the deposited films were characterized as well. The figure below reports an example of this work for the case of iron films grown using Fe(CO)5. In that case, a combination of ISS and AES experiments were used to establish that the metal deposition occurs via the growth of three-dimensional islands, not in a more desirable layer-by-layer mode, and that it is followed by significant carbon incorporation.
LEIS and AES spectra before (left) and after (right) the deposition of an iron film using Fe(CO)5. Because of the different sampling depths of the ISS and AES techniques, the combined results reported here provide a fairly good indication of the morphology of the resulting films. Specifically, it is seen that although the 100 L dose at 500 K used in this example leads to the deposition of an equivalent to seven Fe monolayers (according to the AES data), approximately 30% of the original platinum substrate remains uncovered still (based on the ISS information). This means that the film has grown in a three-dimensional fashion. Moreover, the AES spectrum shows the incorporation of significant amounts of carbon, whereas the ISS trace only identifies the terminal oxygen atom in the carbon monoxide chemisorbed on the topmost layer.