Pollution Abatement
- Kinetics from Molecular Beam Data
- CO Oxidation
- NO Reduction
- Pd Catalysts Supported on Alumina/Zirconia/Lanthana Mixed Oxides
The main purpose of this project has been to characterize the kinetics of environmental catalytic converter processes at a microscopic level using a molecular beam set-up. Our investigation has focused on studying the rates and mechanism of elementary surface steps, including sticking coefficients under realistic catalytic conditions, that is, isothermally, in the presence of other gases, and at the reaction temperatures [F. Zaera, Int. Rev. Phys. Chem. 21 (2002) 433]. At a more fundamental level, these studies are also being used to investigate the deviations from mean-field Wigner-Polanyi kinetics on surfaces, in particular as a consequence of spatially inhomogeneous distributions of adsorbates on surfaces [F. Zaera, Acc. Chem. Res. 35 (2002) 129]. Three reactions have been initially chosen as representative for the performance of the metallic phases in the three-way catalyst, namely: (1) the oxidation of carbon monoxide on platinum; (2) the reduction of nitrogen monoxide by carbon monoxide on rhodium; and (3) the oxidation of alkanes on palladium, platinum, and nickel.
Kinetics from Molecular Beam Data
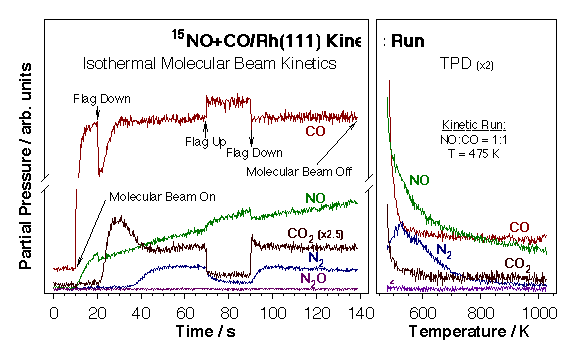
Most of our experimental data in this project has been obtained by using the effusive molecular beam system described in the "Molecular Beams and Nanoreactors" Section. The experimental procedure used in these experiments is illustrated by the data below for the case of NO+CO/Rh(111). A series of actions are taken during these experiments as follows: (1) The molecular beam is turned on with the flag in the intercepting position so the crystal is not yet exposed directly to the beam (t = 10 s). At this point the reactant molecules (NO and CO) are scattered throughout the vacuum chamber, and their partial pressures increase up to new steady-state values. (2) The flag is removed from the path of the beam in order to allow for its direct impingement on the surface (t = 20 s). This causes both a decrease in the partial pressures of the reactants and an increase in the signals of the products proportional to the respective rates of reaction. (3) The system is let to evolve until a steady state is reached, which in general happens within 50 s after the unblocking of the beam. (4) During the steady-state regime the molecular beam is blocked and unblocked deliberately to check on the values of the reaction rates (t = 70 and 90 s, respectively). (5) The beam is turned off to stop the reaction (t = 200 s). (7) The partial pressures of reactants are allowed to return to their background levels, the crystal is cooled down, and the temperature is ramped to record temperature programmed desorption spectra of the relevant species.
Raw data from a typical isothermal kinetic run of the type discussed here. An effusive collimated molecular beam of a 15NO + CO gas mixture is directed onto a clean rhodium (111) surface kept at a constant temperature while the partial pressures of all reactants and products are followed as a function of time. Temperature-programmed desorption (TPD) spectra are then taken for the key species after the isothermal kinetic runs to estimate surface coverages (right panel). Both surface coverages and desorption rates can obtained as a function of time by simple manipulation of these data.CO Oxidation
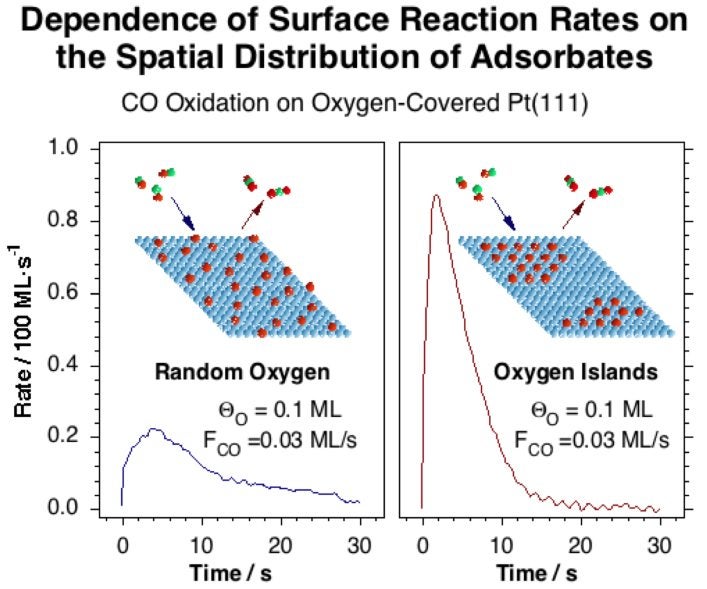
This experimental setup was used first to study the details of the kinetics of CO adsorption on Pt(111) [J. Liu, M. Xu, T. Nordmeyer and F. Zaera, J. Phys. Chem. 99 (1995) 6167)], and then to characterize the oxidation of CO by oxygen on the same surface [J. Liu, M. Xu and F. Zaera, Catal. Lett. 37 (1996) 9; F. Zaera, J. Liu and M. Xu, J. Chem. Phys. 106 (1997) 4204]. It was found that, under certain conditions, the rate of surface recombination of CO with oxygen competes with that of CO adsorption, and that the overall dynamic behavior is fairly complex, since not all the surface oxygen is reactive and the reaction rates not only depend on the coverages of the reactants but also on how the surface is prepared [M. Xu, J. Liu and F. Zaera, J. Chem. Phys. 104 (1996) 8825]. Two kinetically distinct types of oxygen atoms develop during the course of reaction in spite of the fact that they all sit on identical sites at the start of the kinetic runs, suggesting that the reactivity of chemisorbed CO depends on the local oxygen coverage of neighboring sites. We propose that such local arrangements modify the adsorption energy for atomic oxygen, and that this in turn changes the activation energy for the oxidation reaction. More recent experiments have extended our initial studies to Pd surfaces [V. V. Gorodetskii, A. V. Matveev, E. A. Podgornov and F. Zaera, Top. Catal. 32 (2005) 17].
Carbon dioxide production rate during the oxidation of CO at 350 K on two oxygen-covered Pt(111) surfaces prepared by different methods. The initial oxygen and CO coverages are about the same in both cases, but a different kinetic behavior is nevertheless observed because of the difference in the spatial distribution of the oxygen atoms within the surface.NO Reduction
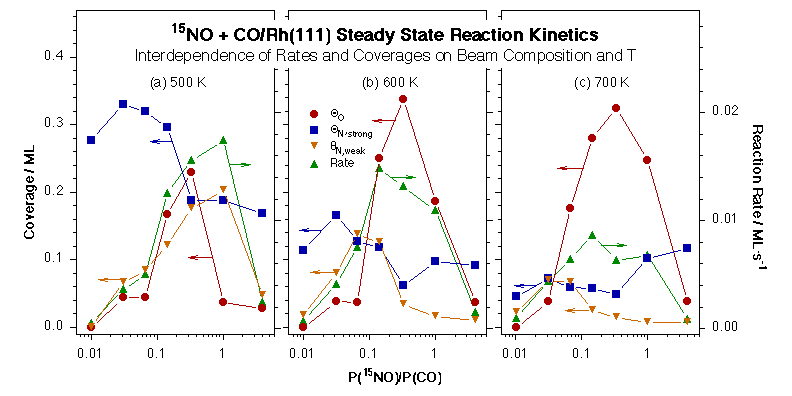
Next we have studied the kinetics of the reduction of NO by CO on Rh(111). At first data was obtained for the thermal chemistry of NO alone on that surface [M. Aryafar and F. Zaera, J. Catal. 175 (1998) 316], after which systematic measurements were performed on the steady-state rates of the catalytic reaction of NO with CO on the Rh(111) surfaces as a function of surface temperature, NO + CO beam composition, and total beam flux [C. S. Gopinath and F. Zaera, J. Catal. 186 (1999) 387]. A maximum in reaction rate was observed between 450 and 900 K, the exact temperature depending on the NO:CO beam ratio, and a synergistic behavior was seen between increasing CO concentrations in the beam and higher surface temperatures. Based on the response of the different signals to the blocking and unblocking of the beam, the rate-limiting step of the overall NO reduction process was proposed to be the production of molecular nitrogen. Surface coverages were measured by CO titration and TPD experiments after the kinetic runs, and used to determine that the NO+CO conversion rate is directly proportional to the coverage of atomic oxygen on the surface. The relation between reaction rates and nitrogen coverages, however, proved to be much more complex.
Oxygen (circles) and strongly- and weakly-held nitrogen (squares and down-pointing triangles, respectively) coverages and reaction rates (up-pointing triangles) as a function of 15NO:CO beam composition from NO+CO/Rh(111) isothermal kinetic experiments at 500 (a), 600 (b), and 700 K (c). A direct dependence of reactions rates is seen in most cases on oxygen coverages, but a negative correlation appears to hold between those rates and the presence of strongly held surface nitrogen atoms.The role of surface nitrogen in the kinetics of the NO + CO conversion reaction on Rh(111) under steady-state catalytic conditions was explored in more detail [F. Zaera and C. S. Gopinath, J. Phys. Chem. B 104 (2000) 3194]. Two types of kinetically different nitrogen atoms have been identified on the surface [F. Zaera and C. S. Gopinath, J. Chem. Phys. 111 (1999) 8088; F. Zaera and C. S. Gopinath, J. Chem. Phys. 116 (2002) 1128] and, in fact, the build-up of a critical nitrogen coverage was determined to be required before molecular nitrogen could be produced. This threshold coverage is quite high at low temperatures, over half a monolayer around 400 K, but decreases abruptly with increasing reaction temperature until becoming almost insignificant above 600 K. In addition, a small amount of extra nitrogen appears to be present on the surface during catalysis but to desorb rapidly after the removal of the gas-phase reactants; it is the coverage of this second labile nitrogen the one that correlates with the rate of NO reduction rate.
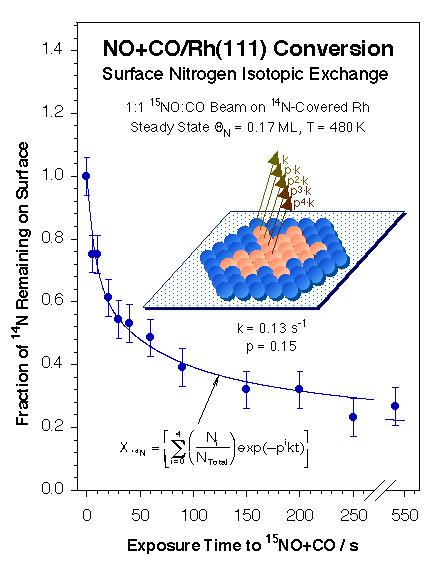
However, isotope-switching experiments have indicated that the two types of kinetically different nitrogen species are in fact in similar adsorption states but display different adsorption energetics because of the effect of their immediate surroundings. Furthermore, as the Figure below shows, the replacement of surface 14N by 15N upon switching the isotopic nitrogen label in the NO (from 14NO to 15NO) was determined to occur via the exclusive formation of 14N15N; no 14N14N was detected in these experiments [F. Zaera and C. S. Gopinath, Chem. Phys. Lett. 332 (2000) 209; F. Zaera and C. S. Gopinath, Phys. Chem. Chem. Phys. 5 (2003) 646]. All these kinetic data can be explained by a model in which the nitrogen atoms form surface islands and react preferentially with new incoming NO molecules to form a N–NO intermediate at the perimeter of those islands [F. Zaera and C. S. Gopinath, J. Mol. Catal. A 167 (2001) 23]. Both Monte Carlo simulations [F. Zaera, S. Wehner, C. S. Gopinath, J. L. Sales, V. Gargiulo and G. Zgrablich, J. Phys. Chem. B 105 (2001) 7771; V. Bustos, C. S. Gopinath, R. Uñac, F. Zaera and G. Zgrablich, J. Chem. Phys. 114 (2001) 10927; V. Bustos, R. Uñac, F. Zaera and G. Zgrablich, J. Chem. Phys. 118 (2003) 9372; L. A. Avalos, V. Bustos, R. Uñac, F. Zaera and G. Zgrablich, J. Mol. Catal. A 228 (2005), 89; L. A. Avalos, V. Bustos, R. Uñac, F. Zaera, G. Zgrablich, J. Phys. Chem. B 110 (2006) 24964; F. Zaera, J. L. Sales, M. V. Gargiulo, M. Ciacera, and G. Zgrablich, J. Phys. Chem. C 111 (2007) 7795], and experiments with adsorbed nitrogen [F. Zaera and C. S. Gopinath, J. Chem. Phys. 116 (2002) 1128; F. Zaera and C. S. Gopinath, Phys. Chem. Chem. Phys. 5 (2003) 646] and with N2O [S. Wehner, M. T. Paffett, and F. Zaera, J. Phys. Chem. B 108 (2004), 18683] corroborate these conclusions. Finally, the effect of oxygen in the gas mixture has been explored [C. S. Gopinath and F. Zaera, J. Catal., 200, 270-287 (2001)].
|
14N and 15N surface coverages during the steady-state conversion of NO as a function of the time a 14N-covered surface is exposed to a 15NO+CO beam. The original 14N is slowly replaced by new 15N as expected, but by following a complex kinetics. The circles represent the experimental data, while the lines are the result of a fit to the data with the islanding model depicted on the right. The surface nitrogen atoms are proposed to form islands of nitrogen atoms whose reactivity depend exponentially on the distance from the perimeter of the island.
|
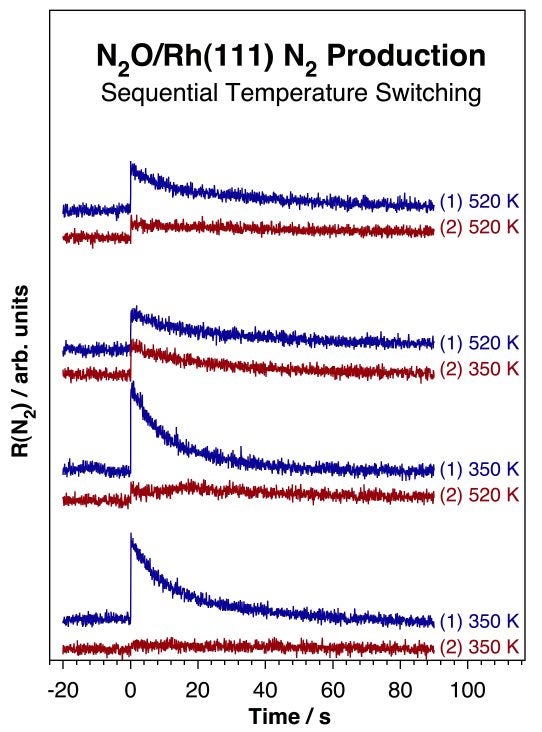
The issue of N2 production via a N–NO intermediate is further complicated by the unusual temperature dependence observed in the kinetics of N2O decomposition [S. Wehner, M. T. Paffett, and F. Zaera, J. Phys. Chem. B 108 (2004) 18683]. The decomposition of pure N2O was determined to occur at temperatures as low as 120 K, to follow first-order kinetics, and to lead to the stoichiometric production of N2(g) and atomic adsorbed oxygen. However, lower rates and total yields are observed with increasing reaction temperatures. In fact, it was shown experimentally that after the rhodium surface is rendered inactive by N2O decomposition at high (520 K) temperatures, significant activity is still possible at lower (350 K) temperatures (see Figure below). Monte Carlo simulations explained these observations by assuming that the surface sites required for the activation of adsorbed N2O increase in size with increasing reaction temperature [R. O. Uñac, V. Bustos, J. Wilson, G. Zgrablich, and F. Zaera, J. Chem. Phys. 125 (2006) 074705].
|
Isothermal molecular beam kinetic data for the decomposition of N2O on a Rh(111) single-crystal surface. Shown are the rates of molecular nitrogen production as a function of time for four sets of double-run experiments. In each pair an initial run was carried out on the clean surface at either one of two temperatures, 350 (two bottom pairs) or 520 (two top pairs) K; the results are reported in the top traces of each pair. A second run was then recorded immediately afterwards, without cleaning the surface, but after changing the temperature. Those results are shown in the lower traces of each pair. The data in this figure indicate that N2O decomposition is slower, produces less N2, and deposits less atomic oxygen on the surface at higher temperatures. It also points to the fact that the inactive surfaces obtained at the end of the N2O conversion at high temperatures can still promote additional decomposition at lower temperatures.
|
Pd Catalysts Supported on Alumina/Zirconia/Lanthana Mixed Oxides
Finally, in a separate project, we have explored, in collaboration with the group of Dr. Sergio Fuentes at UNAM-Ensenada, the use of sol-gel methods for the preparation of more stable palladium-based catalysts supported on alumina-zirconia-lanthana mixed oxides [H. Tiznado, S. Fuentes and F. Zaera, Langmuir 20 (2004) 10490; G. Perez-Osorio, F. Castillon, A. Simakov, H. Tiznado, F. Zaera, and S. Fuentes, Appl. Catal. B 69 (2007) 219].